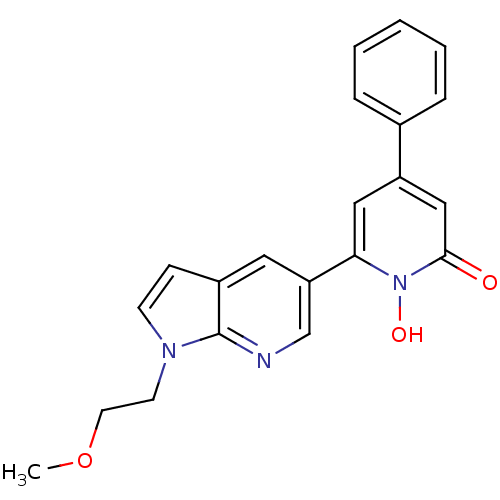
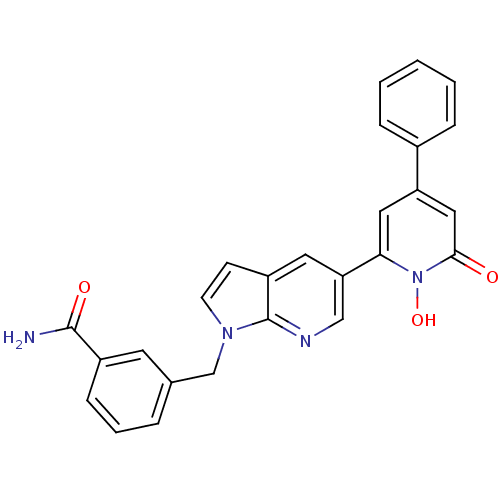
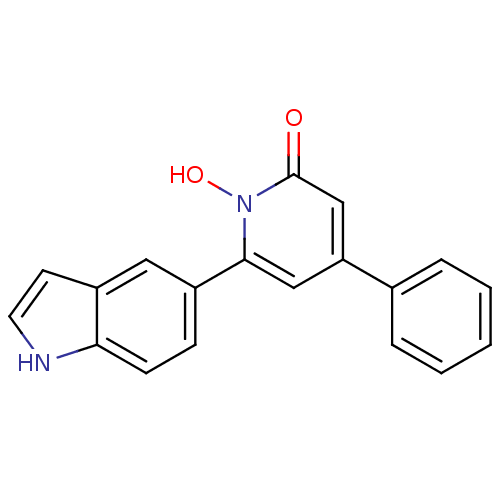
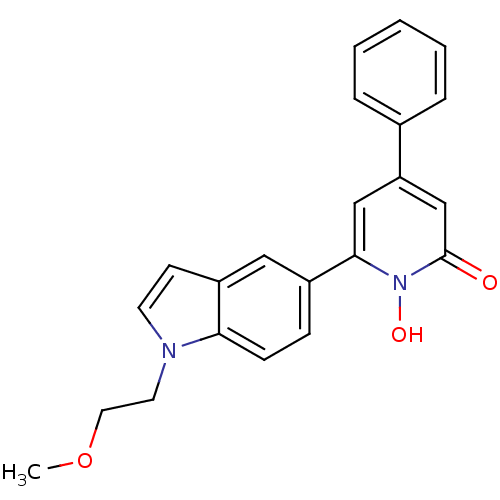
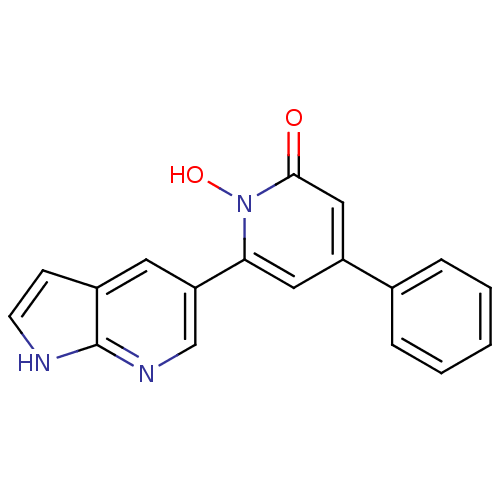
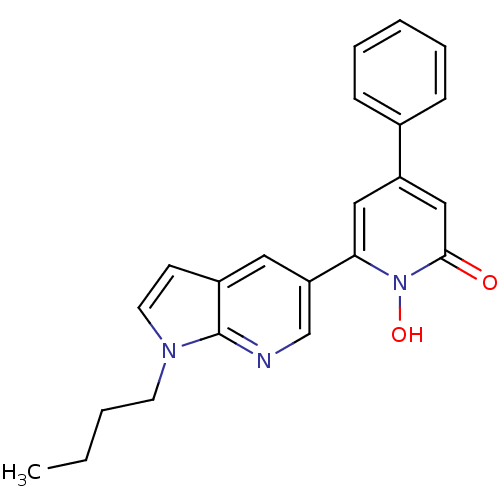
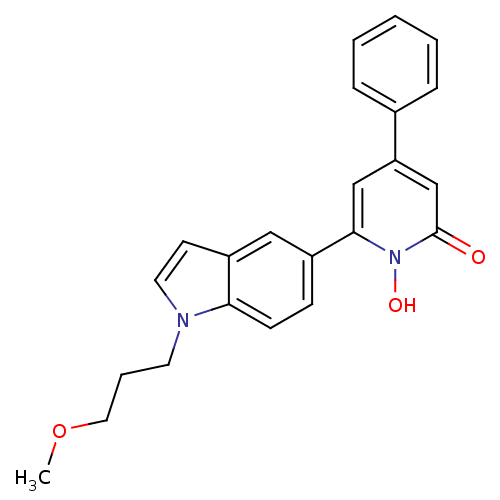
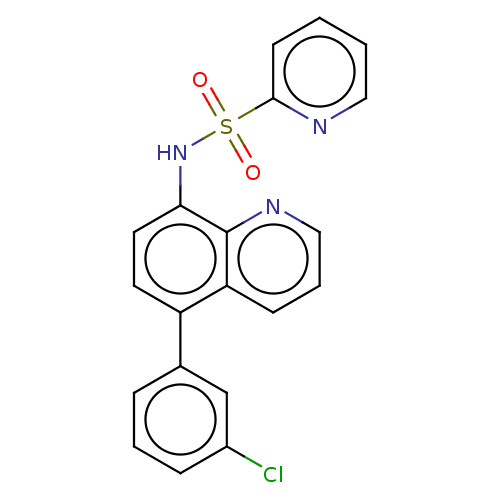
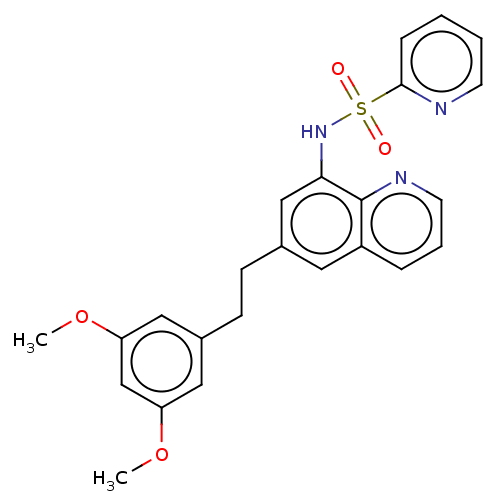
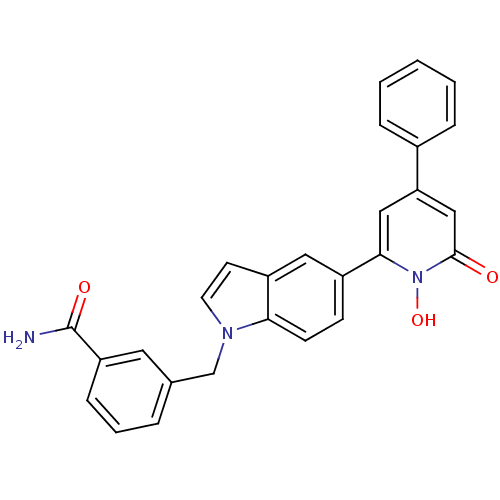
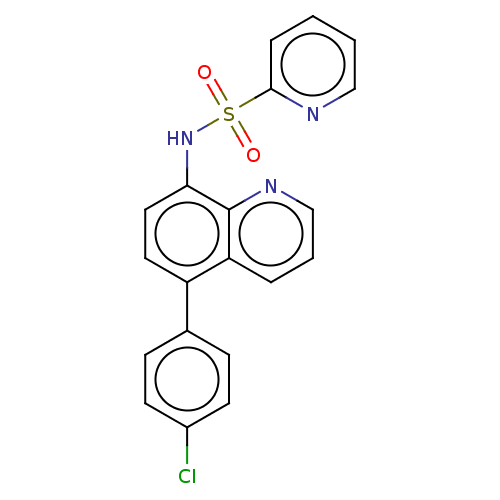
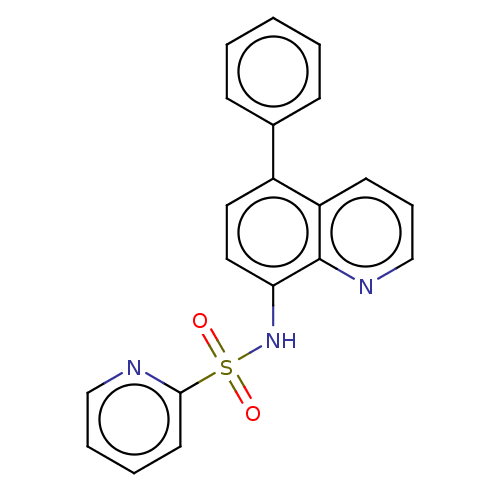
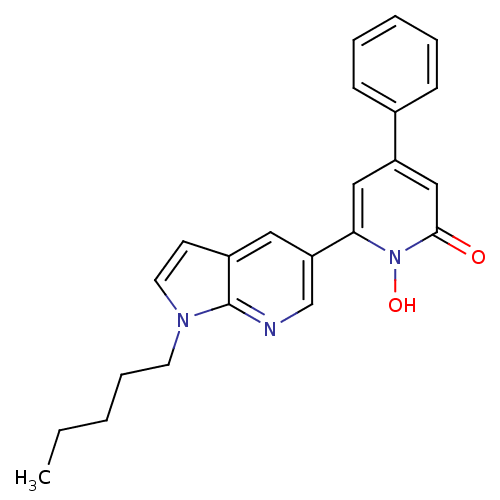
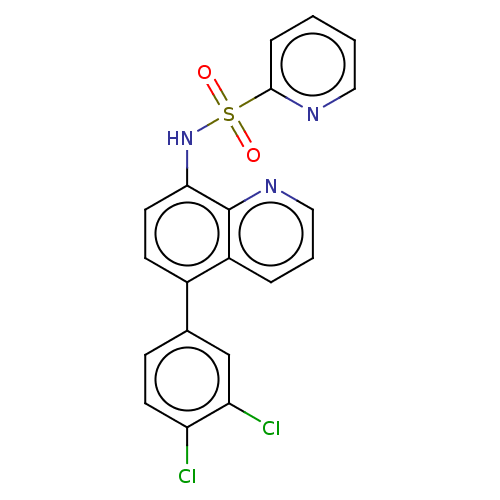
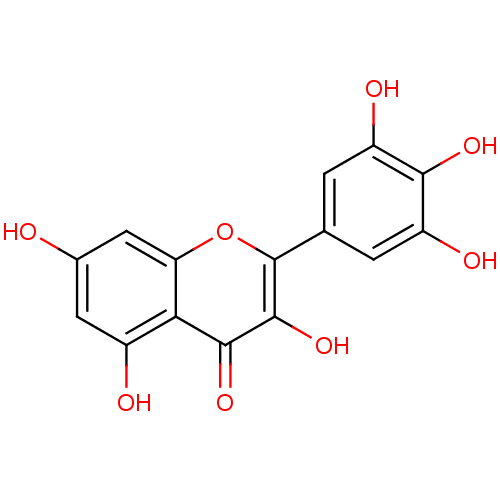
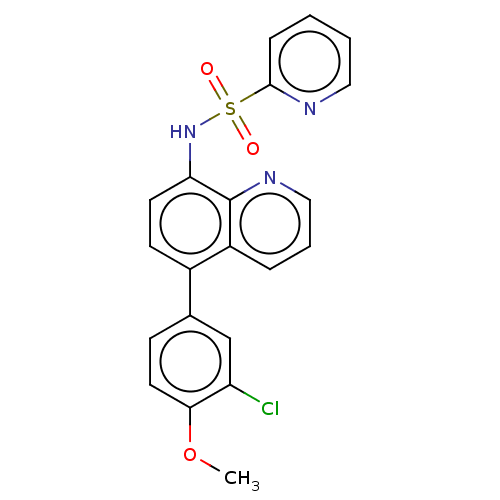
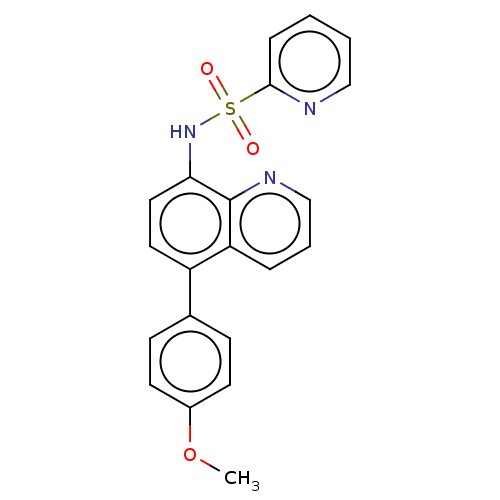
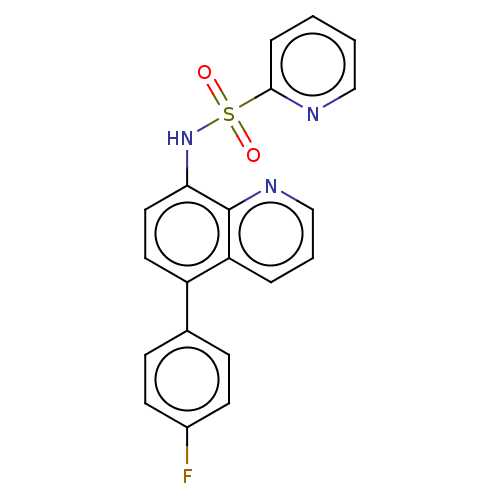
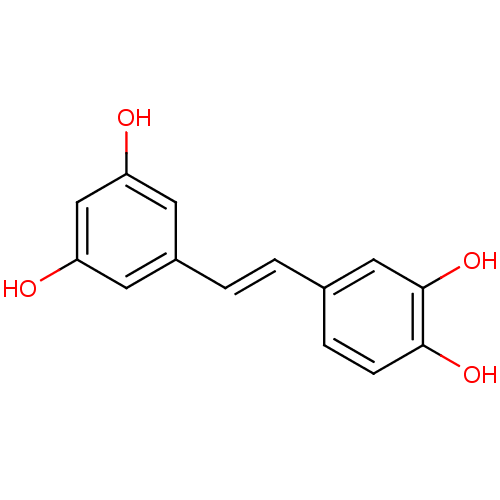
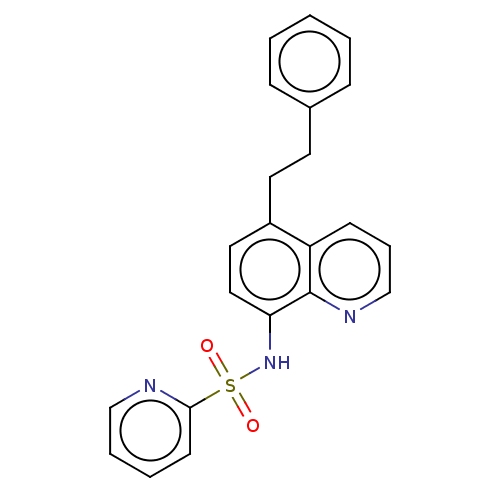
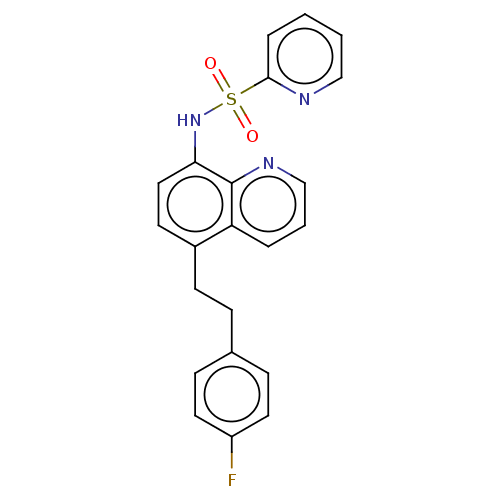
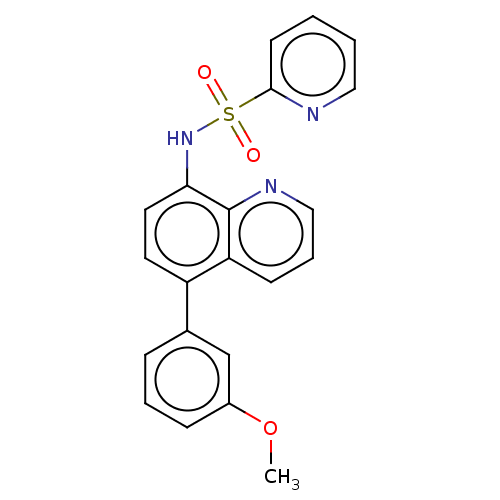
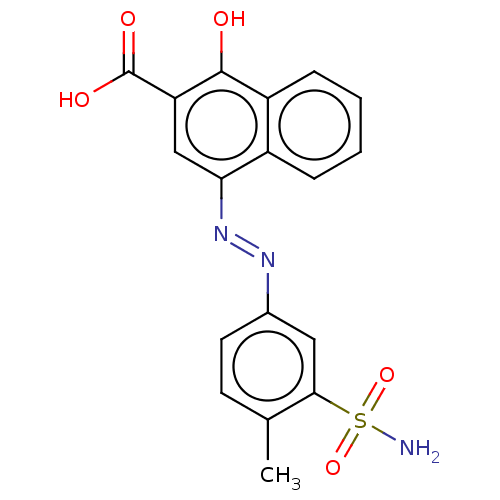
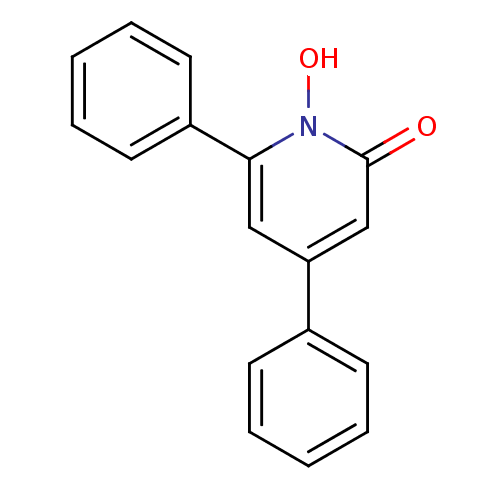
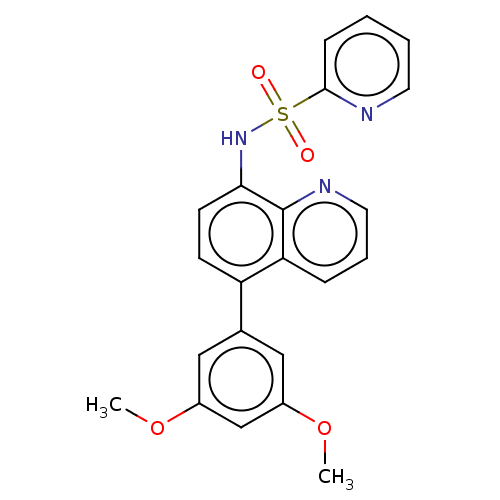
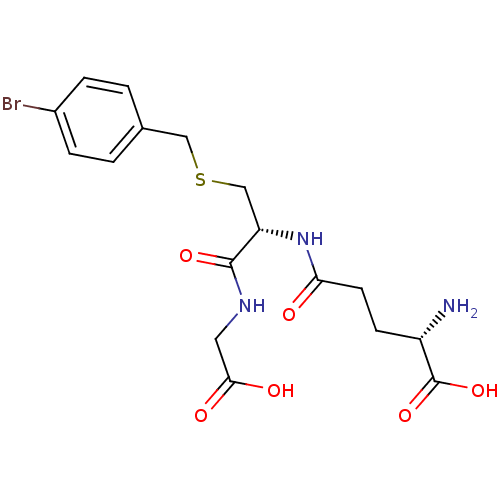
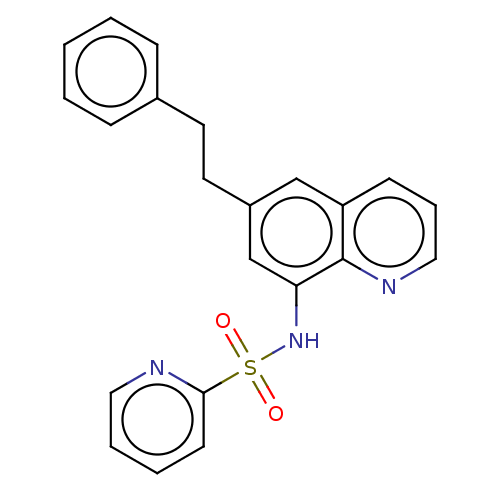
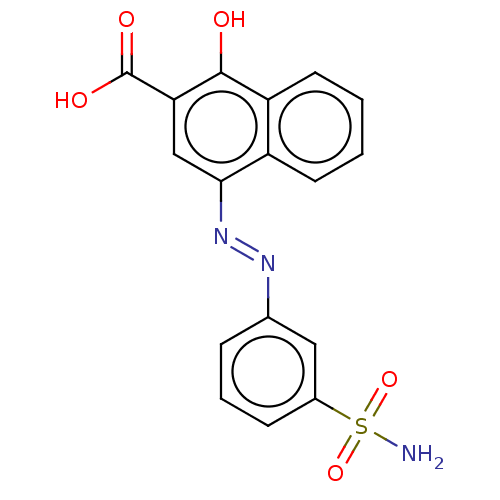
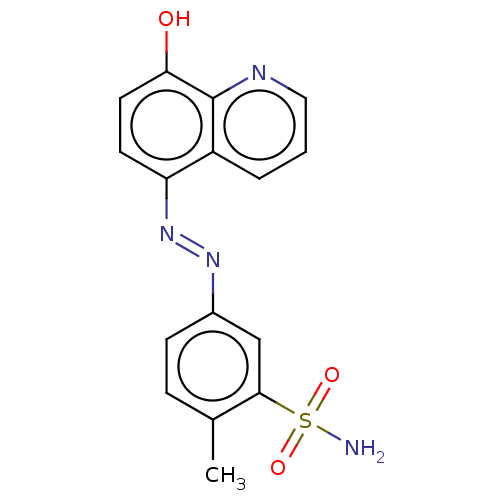
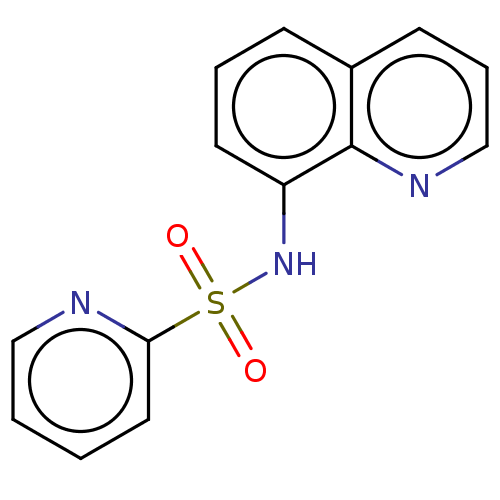
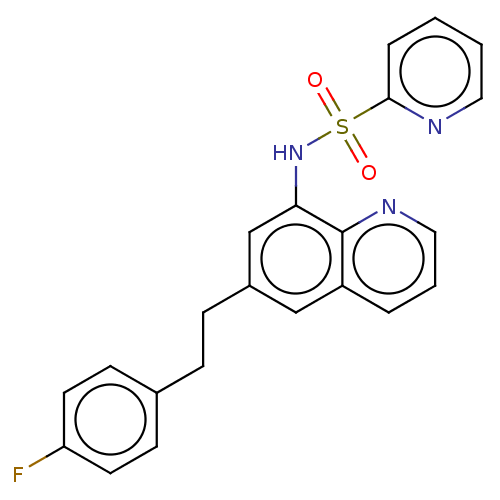
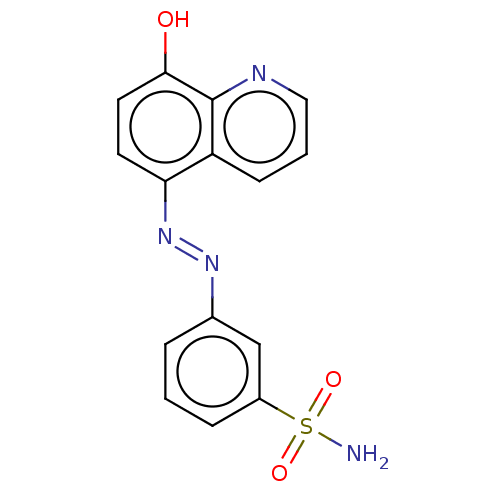
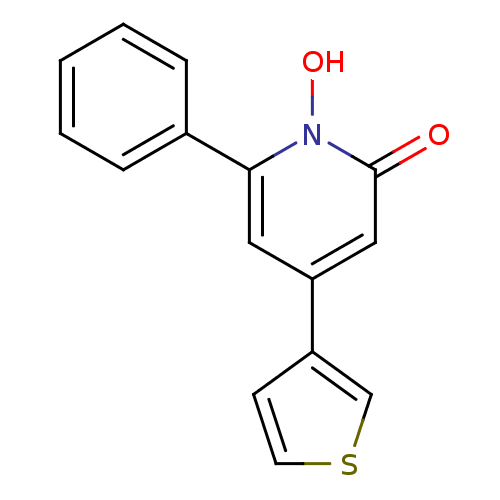
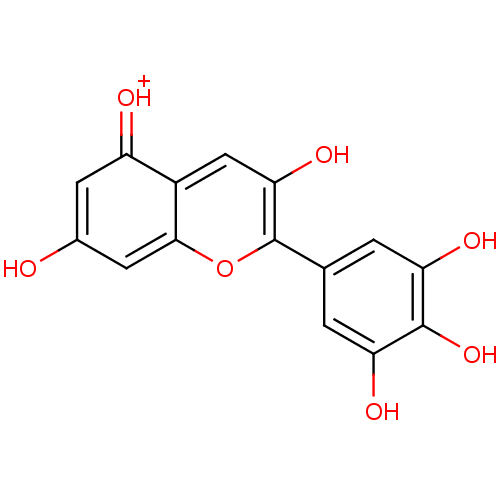
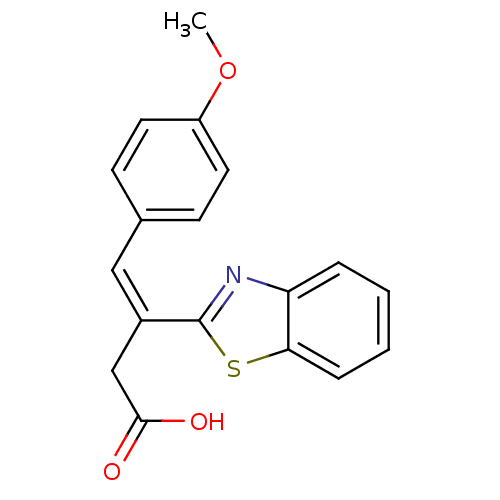
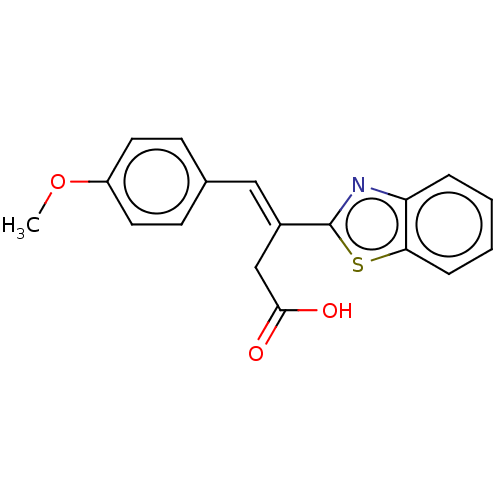
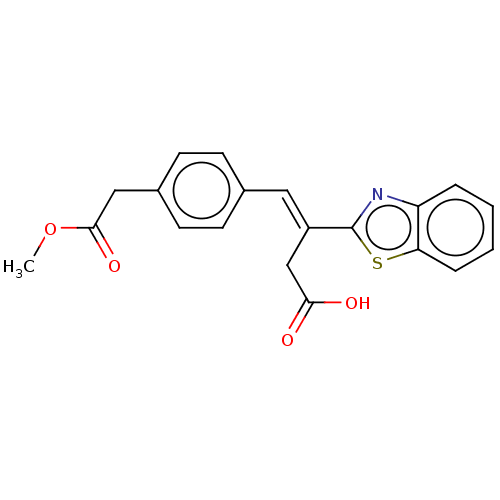
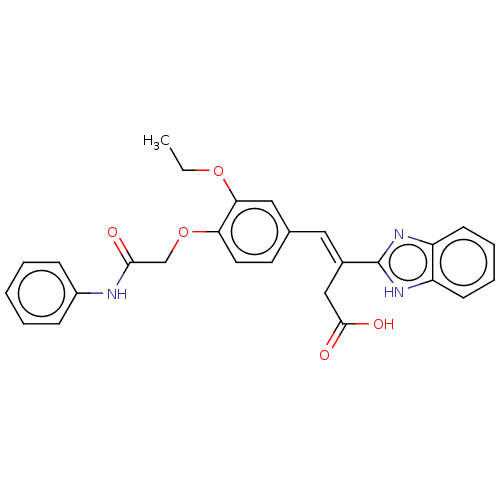
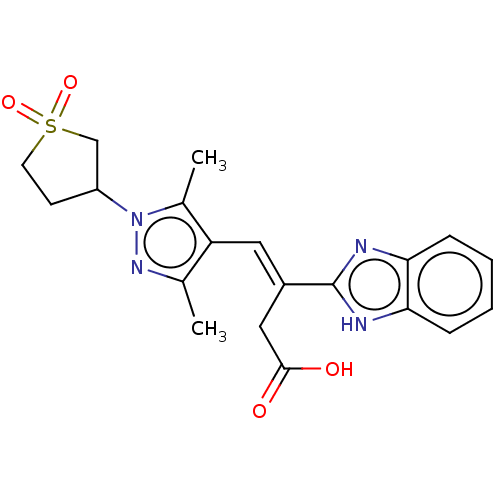
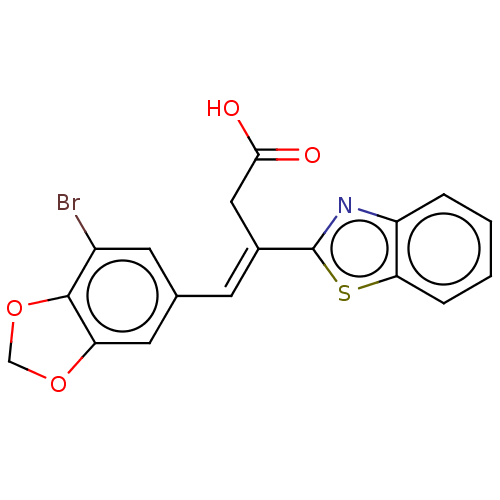
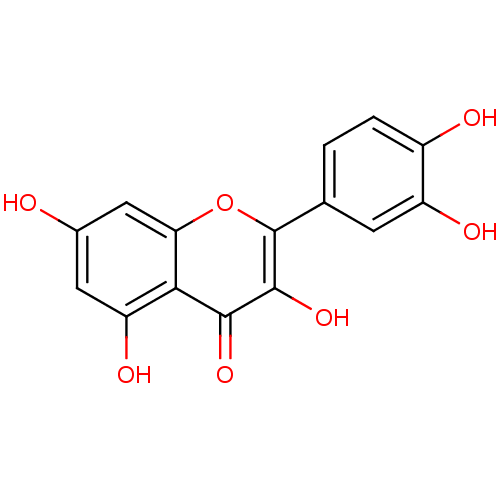
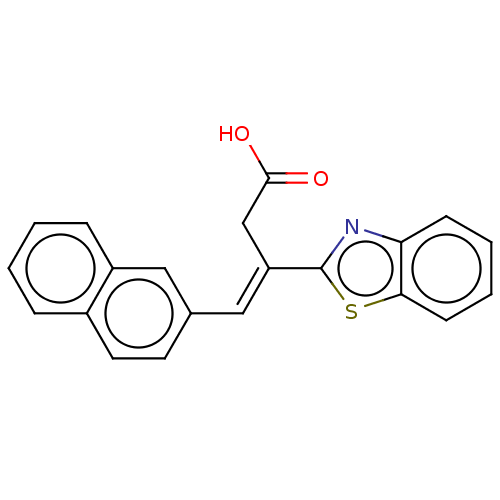
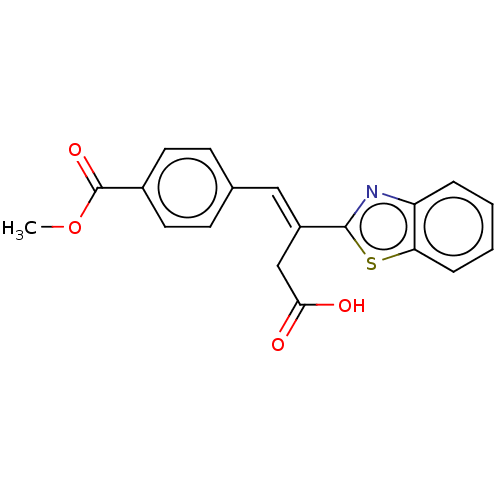
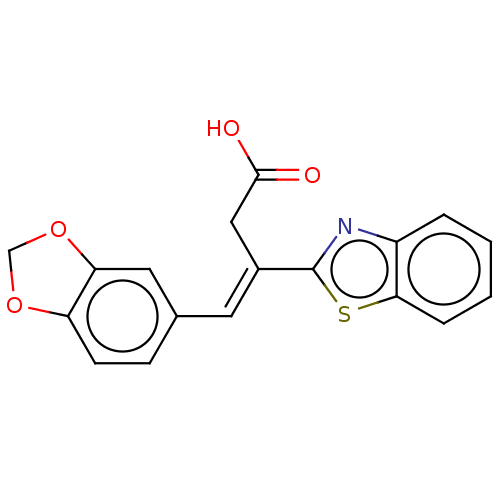
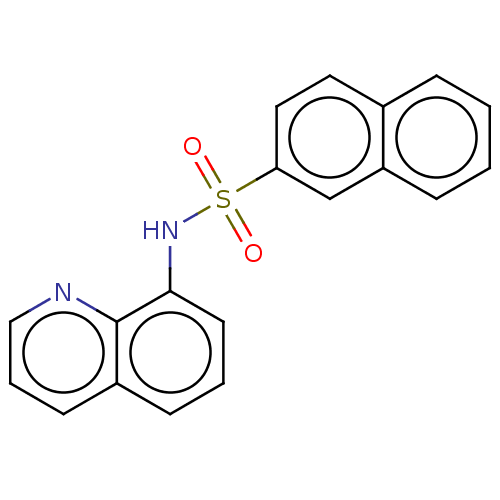
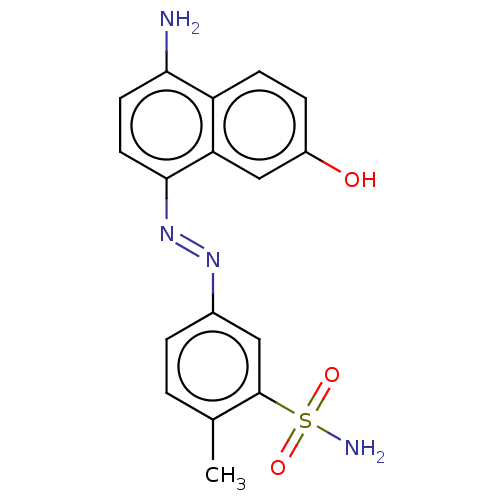
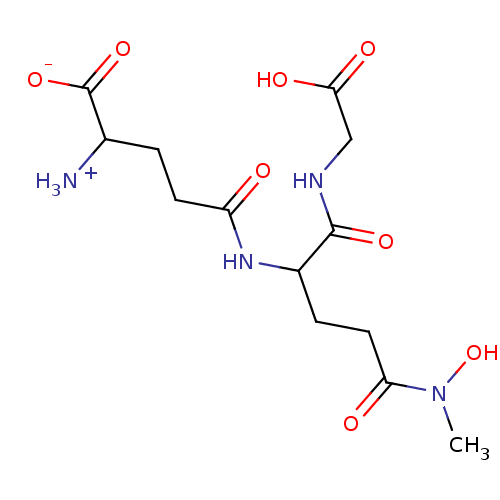
Affinity DataIC50: 11nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 540nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 640nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 760nMAssay Description:Inhibition of human N-terminal His6-tagged GLO1 expressed in baculovirus infected sf21 cells assessed as reduction in S-D-lactoylglutathione formatio...More data for this Ligand-Target Pair
Affinity DataIC50: 790nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 790nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+3nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+3nMAssay Description:Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.19E+3nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 1.19E+3nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 1.21E+3nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.22E+3nMAssay Description:Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.28E+3nMAssay Description:Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.32E+3nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+3nMAssay Description:Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.52E+3nMAssay Description:Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human recombinant glyoxalase 1 assessed as S-D-lactoylglutathione after 15 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.38E+3nMAssay Description:Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.65E+3nMAssay Description:Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using methylglyoxal and reduced glutathione as...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Compound was evaluated for inhibition of Saccharomyces cerevisiae glyoxalase-I, activity is determined with 0.5 mM substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)