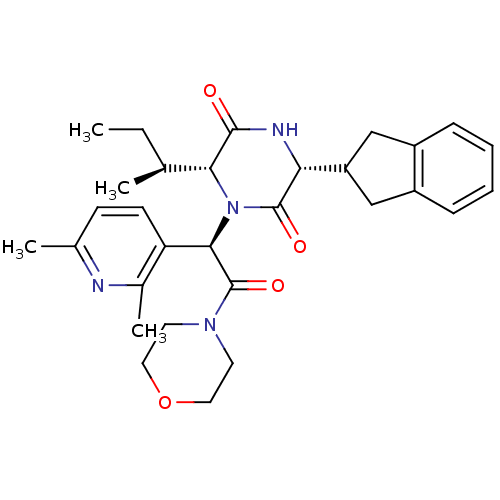
BDBM50384820 EPELSIBAN::GSK557296B
SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1
InChI Key InChIKey=UWHCWRQFNKUYCG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50384820
Found 11 hits for monomerid = 50384820
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 9.20nMAssay Description:Antagonist activity at human OTR expressed in HEK293 cells assessed as decrease in calcium flux measured after 10 mins in presence of vasopressin by ...More data for this Ligand-Target Pair
Affinity DataKi: 3.98E+3nMAssay Description:Displacement of [3H]vasopressin from human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >6.31E+3nMAssay Description:Displacement of [3H]vasopressin from human vasopressin V1a receptorMore data for this Ligand-Target Pair
Affinity DataKi: >7.94E+3nMAssay Description:Displacement of [3H]vasopressin from human vasopressin V1b receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 using 7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP3A4 using diethoxyfluorescein as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair