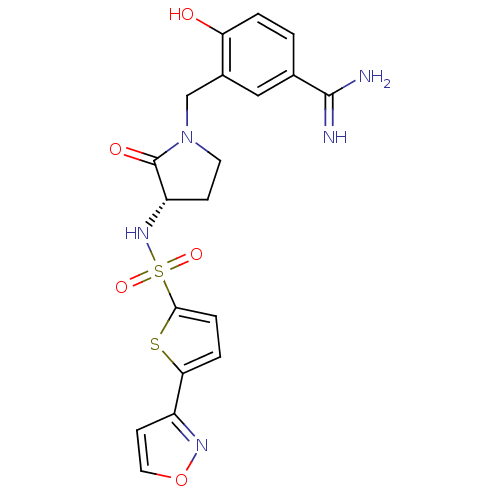
BDBM13306 4-Hydroxy-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-ylsulfonylamino)2-oxopyrrolidin-1-ylmethyl]benzamidine::4-hydroxy-3-{[(3S)-3-{[5-(1,2-oxazol-3-yl)thiophene-2-]sulfonamido}-2-oxopyrrolidin-1-yl]methyl}benzene-1-carboximidamide::Sulfonamidopyrrolidinone 20c
SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3ccc(s3)-c3ccon3)C2=O)c1
InChI Key InChIKey=UPZCVHMIYOGCHJ-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 13306
Found 2 hits for monomerid = 13306
Affinity DataKi: 3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 2.90E+3nMAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair