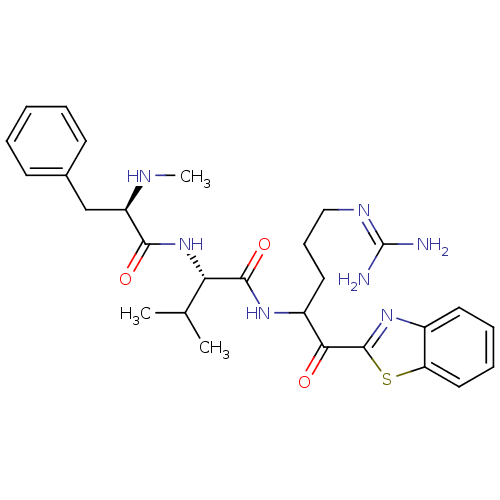
BDBM14085 (2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamido-1-oxopentan-2-yl]-3-methyl-2-[(2R)-2-(methylamino)-3-phenylpropanamido]butanamide::2-ketobenzothiazole 25
SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-c1nc2ccccc2s1
InChI Key InChIKey=GAHMCTXLVGZPSJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 14085
Found 2 hits for monomerid = 14085
Affinity DataKi: 1.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 21nM IC50: 440nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair