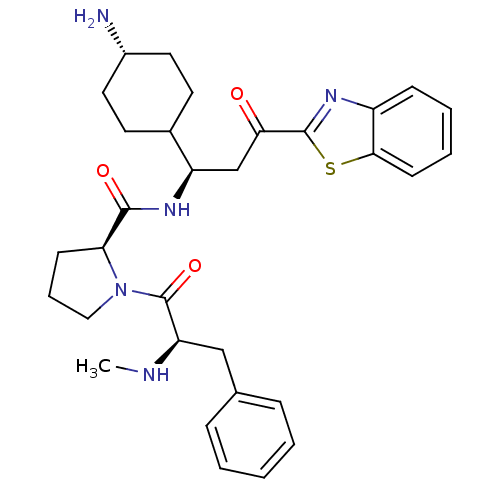
BDBM14133 (2S)-N-[(1R)-1-(4-aminocyclohexyl)-3-(1,3-benzothiazol-2-yl)-3-oxopropyl]-1-[(2R)-2-(methylamino)-3-phenylpropanoyl]pyrrolidine-2-carboxamide::2-ketobenzothiazole 71::The absolute stereochemistry of the carbon thatbears the cyclohexyl ring is unknown::The relative stereochemistry of the cyclohexyl ring is trans
SMILES CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CC(=O)c1nc2ccccc2s1)C1CC[C@H](N)CC1
InChI Key InChIKey=ZDJAWPTWLQRSCD-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 14133
Found 2 hits for monomerid = 14133
Affinity DataIC50: 1.00E+5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+4nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair