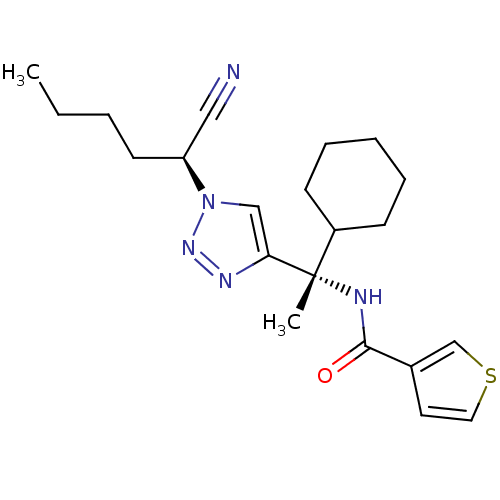
BDBM19483 1, 2, 3 -Triazole Nitrile Inhibitor, 11d::N-[(1S)-1-{1-[(1S)-1-cyanopentyl]-1H-1,2,3-triazol-4-yl}-1-cyclohexylethyl]thiophene-3-carboxamide
SMILES CCCC[C@@H](C#N)n1cc(nn1)[C@@](C)(NC(=O)c1ccsc1)C1CCCCC1
InChI Key InChIKey=ORQKIPRFYXHIBY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 19483
Found 7 hits for monomerid = 19483
Affinity DataIC50: 10nMAssay Description:Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence...More data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -11.0kcal/molepH: 6.1 T: 2°CAssay Description:The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read...More data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:Inhibition of cathepsin S-mediated antigen presentation in B/T hybridoma cells assessed as IL-2 levelMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nM ΔG°: -8.18kcal/molepH: 6.1 T: 2°CAssay Description:The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-7.09kcal/molepH: 6.1 T: 2°CAssay Description:The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-7.09kcal/molepH: 6.1 T: 2°CAssay Description:The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of cathepsin L using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence...More data for this Ligand-Target Pair