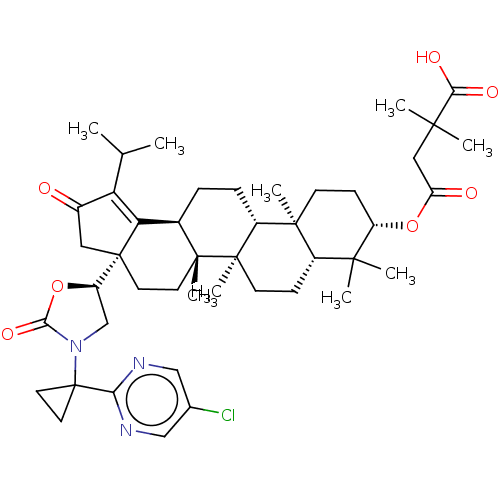
BDBM271434 4- {[(1R,2R,10S,13R,14R, 17S,19R)-5-[(5S)-3-[1-(5- chloropyrimidin-2- yl)cyclopropyl]-2-oxo-1,3- oxazolidin-5-yl]- 1,2,14,18,18- pentamethyl-7-oxo-8- (propan-2- yl)pentacyclo[11.8.0.0{circumflex over ()} {2,10}.0{circumflex over ()}{5,9}.0{circumflex over ()}{14,19}] henicos-8-en-17-yl]oxy}- 2,2-dimethyl-4- oxobutanoic acid::US10064873, Example 60
SMILES [H][C@@]1(CN(C(=O)O1)C1(CC1)c1ncc(Cl)cn1)[C@]12CC(=O)C(C(C)C)=C1[C@@]1([H])CC[C@]3([H])[C@@]4(C)CC[C@H](OC(=O)CC(C)(C)C(O)=O)C(C)(C)[C@]4([H])CC[C@@]3(C)[C@]1(C)CC2
InChI Key InChIKey=CNYBJHQUCXQKAQ-UHFFFAOYSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 271434
Found 2 hits for monomerid = 271434
Affinity DataEC50: 60.2nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160 [V370A](Human immunodeficiency virus type 1)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 8.41E+3nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair