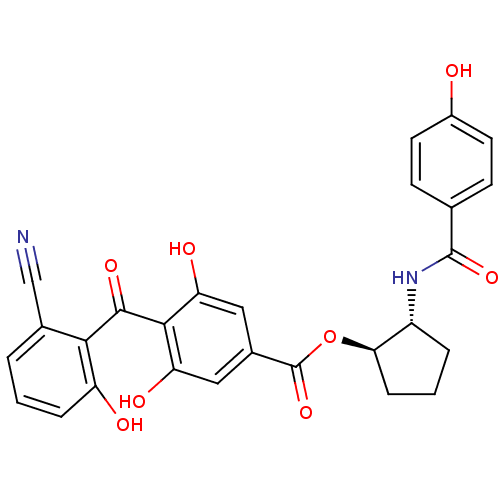
BDBM3232 (1R,2R)-2-[(4-hydroxybenzene)amido]cyclopentyl 4-[(2-cyano-6-hydroxyphenyl)carbonyl]-3,5-dihydroxybenzoate::Modified Benzophenone Carboxylic Acid, Balanol Analog 32::trans-2-[4-(6-Hydroxy-2-nitrilobenzoyl)-3,5-dihydroxybenzoyloxy]-1-(4-hydroxybenzamido)cyclopentane
SMILES Oc1ccc(cc1)C(=O)N[C@@H]1CCC[C@H]1OC(=O)c1cc(O)c(C(=O)c2c(O)cccc2C#N)c(O)c1
InChI Key InChIKey=YWAKWJQTOAUQRV-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 3232
Found 4 hits for monomerid = 3232
Affinity DataIC50: 500nMAssay Description:The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone.More data for this Ligand-Target Pair
Affinity DataIC50: 830nMAssay Description:PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs.More data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:PKC was assayed by quantitating the incorporation of 32P from [gamma-32P]ATP into histone type IIIs.More data for this Ligand-Target Pair