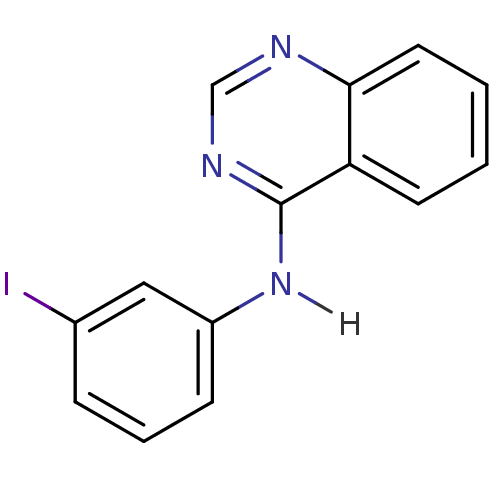
BDBM3265 4-Anilino quinazoline deriv. 16::CHEMBL92825::N-(3-iodophenyl)quinazolin-4-amine
SMILES Ic1cccc(Nc2ncnc3ccccc23)c1
InChI Key InChIKey=LCUWAJDEGKZDMI-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 3265
Found 3 hits for monomerid = 3265
Affinity DataIC50: 80nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair