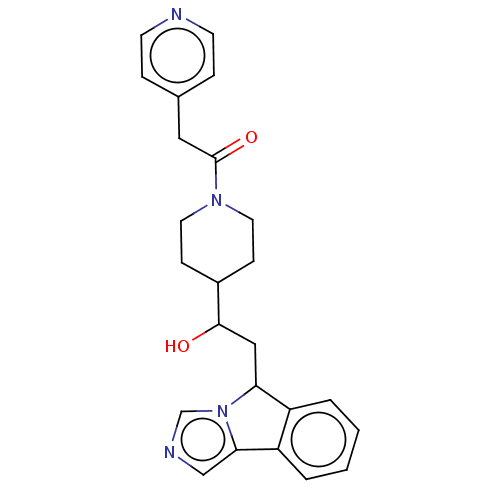
BDBM370517 1-(4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol- 5-yl)ethyl)piperidin-1-yl)-2-(pyridin-4- yl)ethanone::US10233190, Example 1437
SMILES OC(CC1c2ccccc2-c2cncn12)C1CCN(CC1)C(=O)Cc1ccncc1
InChI Key InChIKey=ADXRHXZIAQWYPA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 370517
Found 7 hits for monomerid = 370517
Affinity DataIC50: 230nMAssay Description:Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.30E+3nMAssay Description:Inhibition of recombinant human IDO1 expressed in T-REx-293 cells assessed as reduction in kynurenine level measured after 16 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair