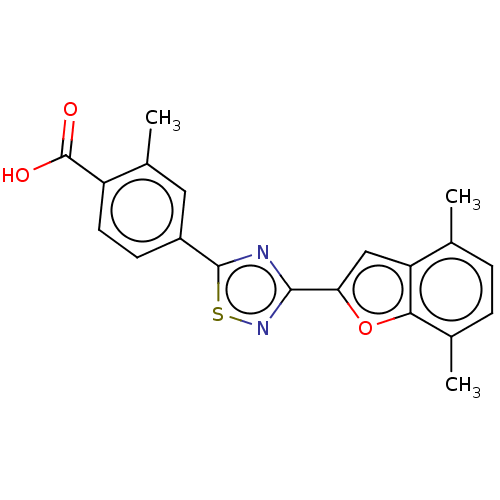
BDBM458285 US10752616, Code No. BHBA-043
SMILES Cc1cc(ccc1C(O)=O)-c1nc(ns1)-c1cc2c(C)ccc(C)c2o1
InChI Key InChIKey=CLIQILUTHAIRCH-UHFFFAOYSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 458285
Found 3 hits for monomerid = 458285
Affinity DataEC50: 5.20nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 44nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair