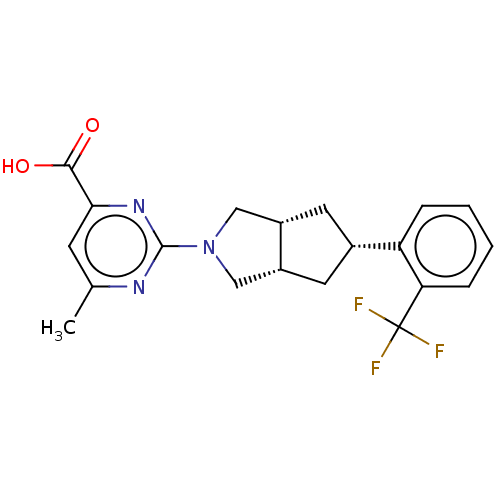
BDBM50104156 CHEMBL3594232
SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)c1nc(C)cc(n1)C(O)=O)c1ccccc1C(F)(F)F
InChI Key InChIKey=XODSGAMMDBEEFN-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50104156
Found 8 hits for monomerid = 50104156
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]-retinol from RBP4 (unknown origin) by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of retinol-induced interaction of bacterially expressed MBP-tagged RBP4 (unknown origin) with Eu3+ cryptate labeled TTR by HTRF assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
Albany College of Pharmacy and Health Sciences
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Agonist activity at PPARgamma (unknown origin) assessed as inhibition by agonist-induced corepressor NCoR release assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Columbia University Medical Center
Curated by ChEMBL
Columbia University Medical Center
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG expressed in HEK293 cells by patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair