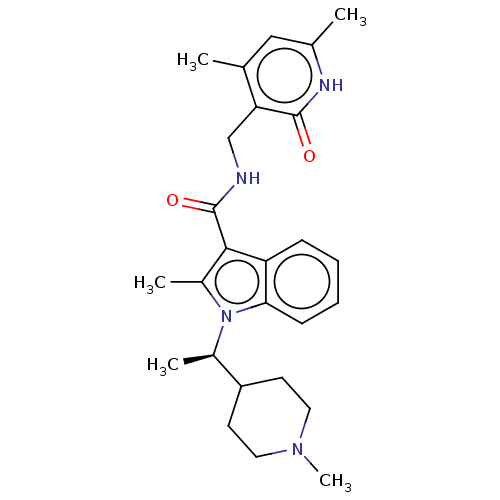
BDBM50110358 CHEMBL3605454
SMILES C[C@H](C1CCN(C)CC1)n1c(C)c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2ccccc12
InChI Key InChIKey=LWNTVQSWMPMEHZ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50110358
Found 3 hits for monomerid = 50110358
Affinity DataIC50: 7nMAssay Description:Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based methodMore data for this Ligand-Target Pair
Affinity DataIC50: 568nMAssay Description:Inhibition of EZH2 in human HeLa cells assessed as reduction in H3K27me3 levels incubated for 72 hrs by ELISA methodMore data for this Ligand-Target Pair