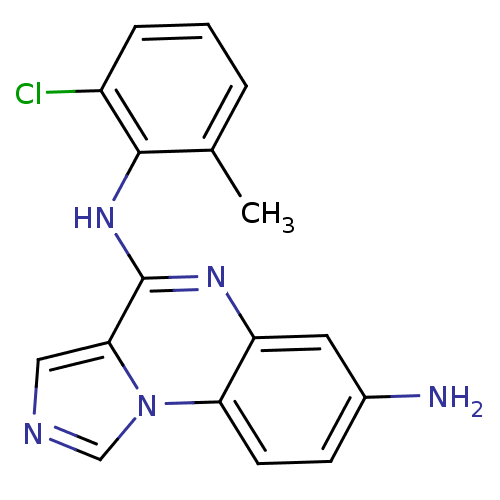
BDBM50120136 CHEMBL83008::N*4*-(2-Chloro-6-methyl-phenyl)-imidazo[1,5-a]quinoxaline-4,7-diamine::N4-(2-chloro-6-methylphenyl)imidazo[1,5-a]quinoxaline-4,7-diamine
SMILES Cc1cccc(Cl)c1Nc1nc2cc(N)ccc2n2cncc12
InChI Key InChIKey=VOWKXPVQYBNEJS-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50120136
Found 3 hits for monomerid = 50120136
Affinity DataIC50: 21nMAssay Description:Evaluated for inhibition of human p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme.More data for this Ligand-Target Pair