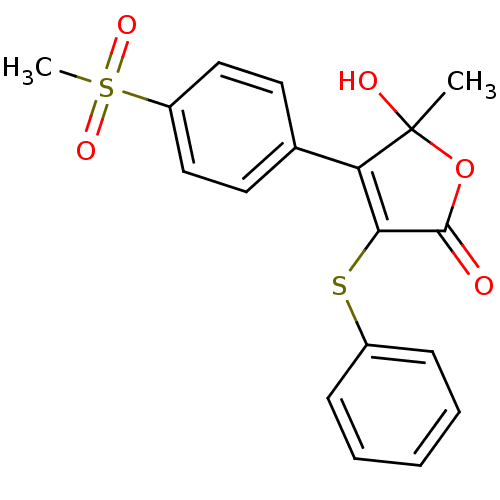
BDBM50125629 5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-phenylsulfanyl-5H-furan-2-one::CHEMBL18377
SMILES CC1(O)OC(=O)C(Sc2ccccc2)=C1c1ccc(cc1)S(C)(=O)=O
InChI Key InChIKey=BJXHPQCXFDPUCE-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50125629
Found 3 hits for monomerid = 50125629
TargetProstaglandin G/H synthase 2(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells.More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells.More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory concentration was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assayMore data for this Ligand-Target Pair