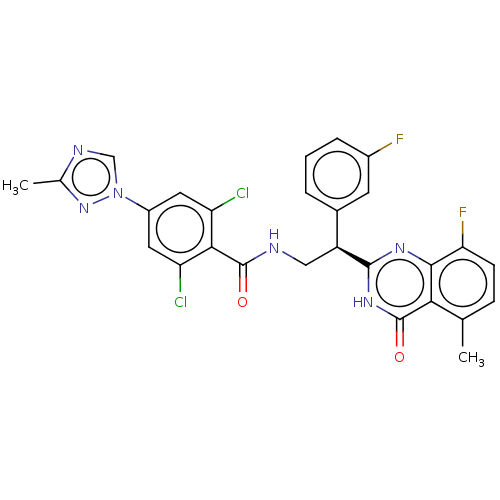
BDBM50125979 CHEMBL3627899
SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2nc3c(F)ccc(C)c3c(=O)[nH]2)c(Cl)c1
InChI Key InChIKey=JKMOLEWKZMQMRF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50125979
Found 7 hits for monomerid = 50125979
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.28E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.44E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrateMore data for this Ligand-Target Pair
Affinity DataKi: >2.89E+4nMAssay Description:Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrateMore data for this Ligand-Target Pair