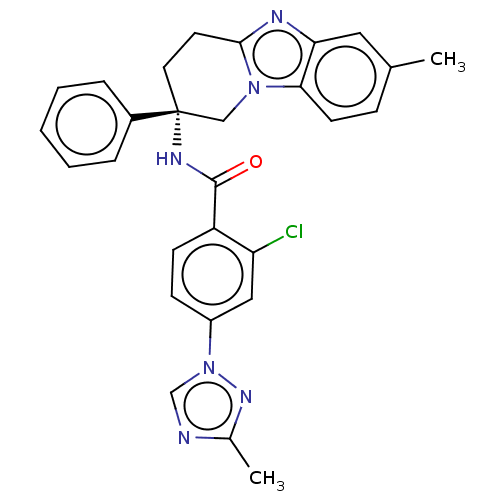
BDBM50126923 CHEMBL3628832::US10351558, Example 67
SMILES Cc1ncn(n1)-c1ccc(C(=O)N[C@@]2(CCc3nc4cc(C)ccc4n3C2)c2ccccc2)c(Cl)c1
InChI Key InChIKey=FUFZNIPCWZLVGH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50126923
Found 4 hits for monomerid = 50126923
Affinity DataKi: 5.90nMAssay Description:Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.04E+3nMAssay Description:Inhibition of human F10a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assayMore data for this Ligand-Target Pair