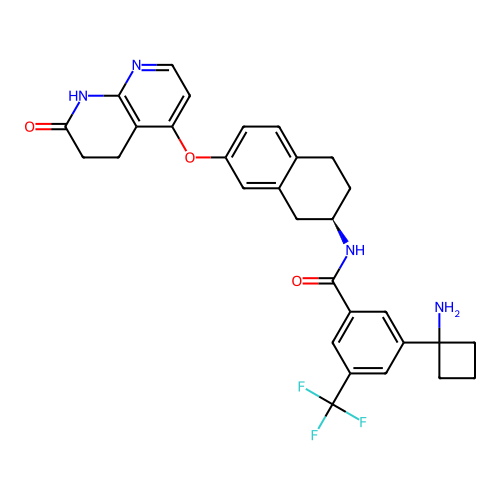
BDBM50139149 CHEMBL3765012
SMILES NC1(CCC1)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1
InChI Key InChIKey=PKHMAUVAHJMDLV-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50139149
Found 3 hits for monomerid = 50139149
Affinity DataIC50: 1.80nMAssay Description:Inhibition of B-Raf V600E mutant (unknown origin) preincubated for 20 mins followed by addition of 2 microM Biotin-DRGFPRARYRARTTNYNSSRSRFYSGFNSRPRGR...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of B-Raf V600E mutant in human A375 cell line assessed as inhibition of ERK phosphorylation for 3 hrs with compound by ELISAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of human ERG using tracer red fluorescent probeMore data for this Ligand-Target Pair