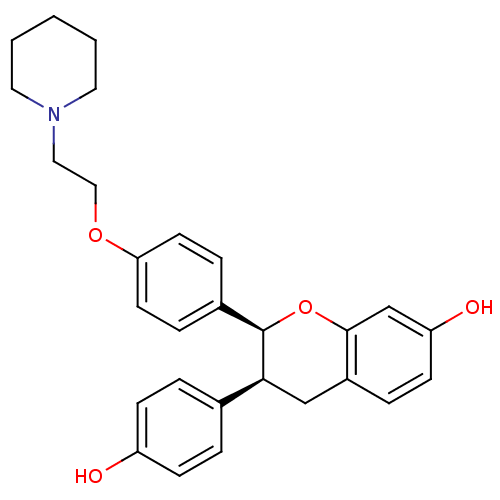
BDBM50141155 (2S,3S)-3-(4-Hydroxy-phenyl)-2-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-chroman-7-ol::CHEMBL30439
SMILES Oc1ccc(cc1)[C@@H]1Cc2ccc(O)cc2O[C@@H]1c1ccc(OCCN2CCCCC2)cc1
InChI Key InChIKey=KBMDZABSIZNFOF-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50141155
Found 4 hits for monomerid = 50141155
Affinity DataIC50: 6.70nMAssay Description:Binding affinity towards human recombinant Estrogen receptor alpha was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Binding affinity against human estrogen receptor alpha in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Binding affinity towards human recombinant Estrogen receptor beta was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Binding affinity against human estrogen receptor beta (ER beta) in competitive binding assayMore data for this Ligand-Target Pair