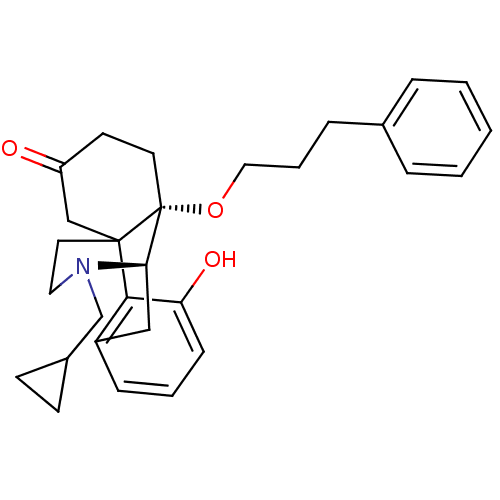
BDBM50148076 17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy)-(1R,9R,10S)-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-trien-13-one::CHEMBL325347
SMILES Oc1cccc2C[C@H]3N(CC4CC4)CCC4(CC(=O)CC[C@@]34OCCCc3ccccc3)c12
InChI Key InChIKey=IWZLUXJMEHSPFL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50148076
Found 6 hits for monomerid = 50148076
Affinity DataEC50: 3.5nMAssay Description:Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor mu 1 expre...More data for this Ligand-Target Pair
Affinity DataEC50: 4.5nMAssay Description:Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor kappa 1 ex...More data for this Ligand-Target Pair
Affinity DataEC50: 7.90nMAssay Description:Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human opioid receptor delta 1 ex...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 5.06E+3nMAssay Description:Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 5.84E+3nMAssay Description:Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranesMore data for this Ligand-Target Pair