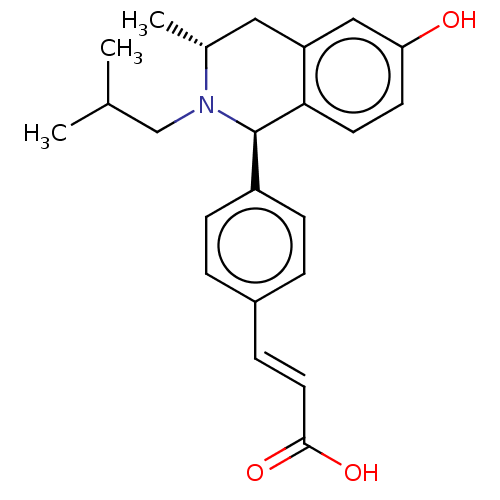
BDBM50153658 CHEMBL3775378
SMILES C[C@@H]1Cc2cc(ccc2[C@H](N1CC(C)C)c3ccc(cc3)/C=C/C(=O)O)O
InChI Key InChIKey=WHZIOQODOSOYPX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50153658
Found 6 hits for monomerid = 50153658
Affinity DataIC50: 1.30nMAssay Description:Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human recombinant mu1 opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant human bradykinin receptor 2 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of recombinant human adenosine receptor A1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG tail current by plate-based planar patch clamp systemMore data for this Ligand-Target Pair