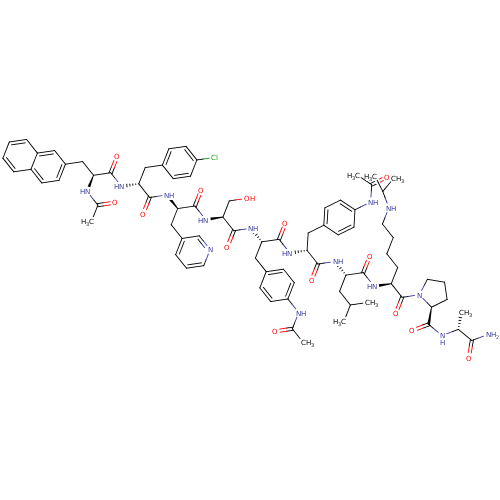
BDBM50209585 CHEMBL414344::[Ncy(2-naphthyl)1]acyline
SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc(NC(C)=O)cc1)NC(=O)[C@H](Cc1ccc(NC(C)=O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc1cccnc1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H](C)C(N)=O
InChI Key InChIKey=ZWNUQDJANZGVFO-UHFFFAOYSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50209585
Found 1 hit for monomerid = 50209585
Affinity DataIC50: 2.20nMAssay Description:Antagonist activity at human GnRHR expressed in HEK293 cells assessed as inhibition of GnRH-induced luciferase response by reporter gene assayMore data for this Ligand-Target Pair