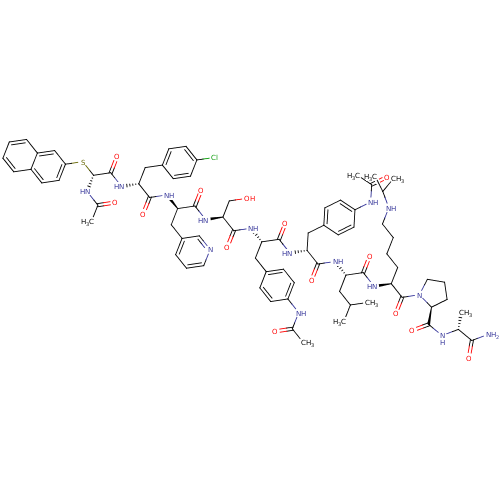
BDBM50209594 CHEMBL227861::[D-Ncy(2-naphthyl)1]acyline
SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc(NC(C)=O)cc1)NC(=O)[C@H](Cc1ccc(NC(C)=O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H](Cc1cccnc1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](NC(C)=O)Sc1ccc2ccccc2c1)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H](C)C(N)=O
InChI Key InChIKey=JRWSZLDRIJBUBZ-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50209594
Found 2 hits for monomerid = 50209594
Affinity DataIC50: 0.730nMAssay Description:Antagonist activity at human GnRHR expressed in HEK293 cells assessed as inhibition of GnRH-induced luciferase response by reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Antagonist activity at rat GnRHR expressed in HEK293 cells assessed as inhibition of GnRH-induced luciferase response by reporter gene assayMore data for this Ligand-Target Pair