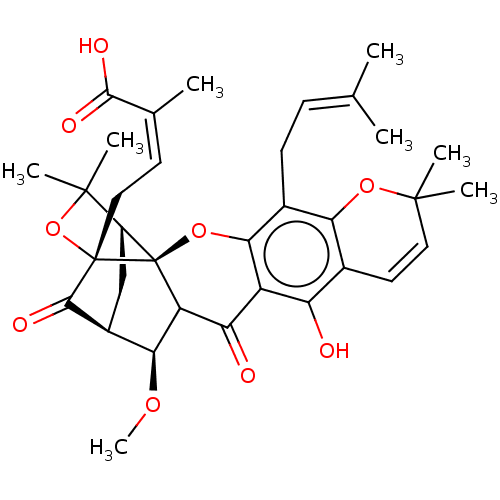
BDBM50256791 CHEMBL4090412
SMILES [H][C@@]12[#6][C@@]3([H])[#6@H](-[#8]-[#6])-[#6]4-[#6](=O)-c5c(-[#8])c6-[#6]=[#6]C([#6])([#6])[#8]-c6c(-[#6]\[#6]=[#6](\[#6])-[#6])c5-[#8][C@@]14[C@@]([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)([#8]C2([#6])[#6])[#6]3=O
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50256791
Found 2 hits for monomerid = 50256791
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Gyeongsang National University
Curated by ChEMBL
Gyeongsang National University
Curated by ChEMBL
Affinity DataIC50: 4.44E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Gyeongsang National University
Curated by ChEMBL
Gyeongsang National University
Curated by ChEMBL
Affinity DataKi: 1.75E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysisMore data for this Ligand-Target Pair