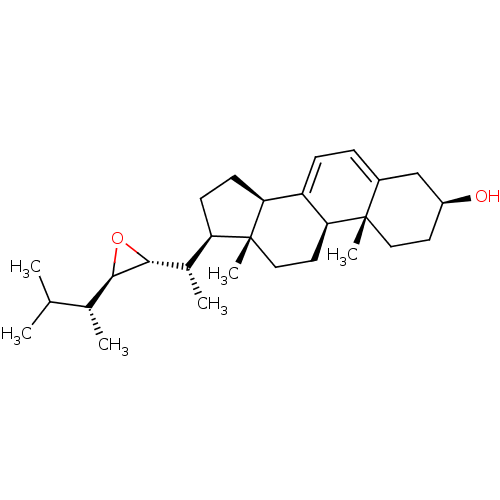
BDBM50257929 CHEMBL4097209
SMILES [H][C@@]1(O[C@]1([H])[C@@H](C)[C@@]1([H])CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)C(C)C
InChI Key InChIKey=QQLHWAKALUIDKJ-UHFFFAOYSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50257929
Found 2 hits for monomerid = 50257929
Affinity DataEC50: 4.11E+3nMAssay Description:Activation of GAL4 fused human LXR alpha expressed in HEK293 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 7.20E+3nMAssay Description:Activation of GAL4 fused human LXR beta expressed in HEK293 cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair