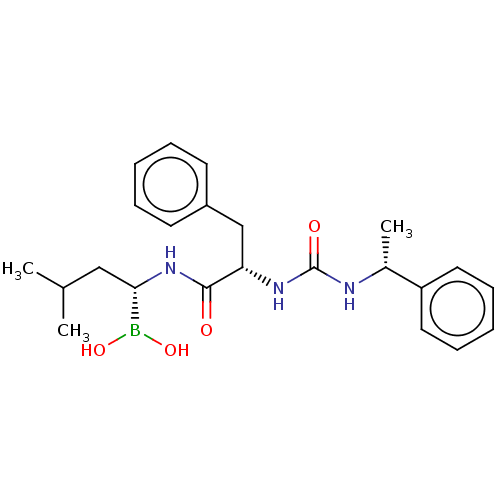
BDBM50259655 CHEMBL4080306
SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@H](C)c1ccccc1)B(O)O
InChI Key InChIKey=NUYQIFLRVLIZGY-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50259655
Found 3 hits for monomerid = 50259655
Affinity DataIC50: 10nMAssay Description:Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy...More data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysisMore data for this Ligand-Target Pair