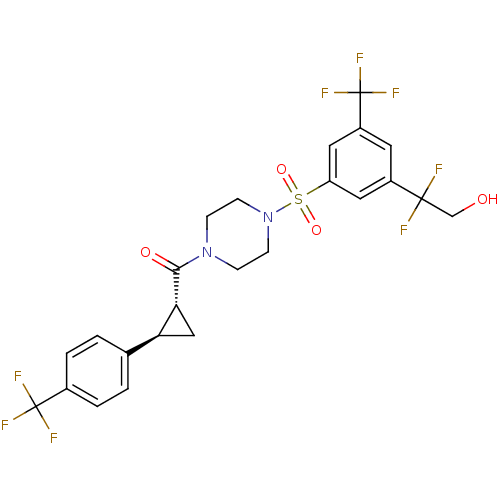
BDBM50267652 (4-(3-(1,1-difluoro-2-hydroxyethyl)-5-(trifluoromethyl)phenylsulfonyl)piperazin-1-yl)((1R,2R)-2-(4-(trifluoromethyl)phenyl)cyclopropyl)methanone::CHEMBL519389
SMILES OCC(F)(F)c1cc(cc(c1)S(=O)(=O)N1CCN(CC1)C(=O)[C@@H]1C[C@H]1c1ccc(cc1)C(F)(F)F)C(F)(F)F
InChI Key InChIKey=STLODSLSZTWODE-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50267652
Found 3 hits for monomerid = 50267652
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB1R expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human ERG by MK-0499 binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2R expressed in CHO cellsMore data for this Ligand-Target Pair