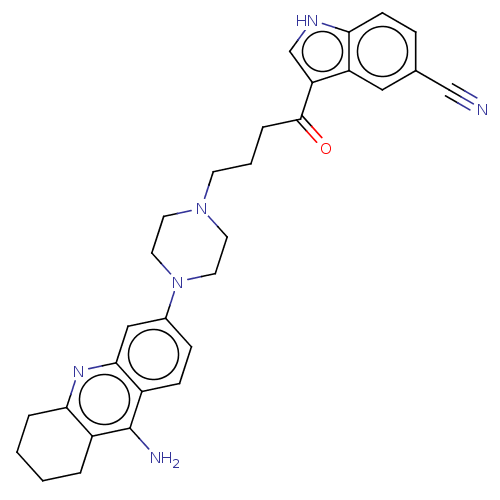
BDBM50272629 CHEMBL4127877
SMILES Nc1c2CCCCc2nc2cc(ccc12)N1CCN(CCCC(=O)c2c[nH]c3ccc(cc23)C#N)CC1
InChI Key InChIKey=VSOMMGVYTLABPP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50272629
Found 3 hits for monomerid = 50272629
Target5-hydroxytryptamine receptor 1A(Human)
East China University of Science and Technology
Curated by ChEMBL
East China University of Science and Technology
Curated by ChEMBL
Affinity DataEC50: 567nMAssay Description:Agonist activity at human 5-HT1A receptor expressed in HEK293 cells after 60 mins by Eu-cAMP solution based ultra LANCE assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.98E+3nMAssay Description:Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate after 20 mins by by Ellman's methodMore data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Rat)
East China University of Science and Technology
Curated by ChEMBL
East China University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate after 20 mins by by Ellman's methodMore data for this Ligand-Target Pair