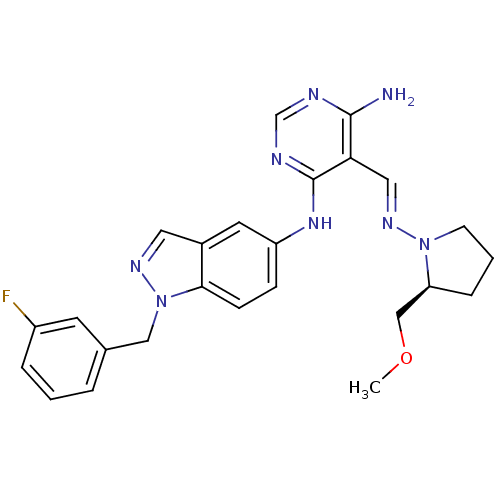
BDBM50272882 (S)-N4-(1-(3-fluorobenzyl)-1H-indazol-5-yl)-5-((2-(methoxymethyl)pyrrolidin-1-ylimino)methyl)pyrimidine-4,6-diamine::CHEMBL456758
SMILES COC[C@@H]1CCCN1\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1
InChI Key InChIKey=ITVLSWHERKANSP-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50272882
Found 3 hits for monomerid = 50272882
TargetReceptor tyrosine-protein kinase erbB-2(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of human ErbB2More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of human EGFR expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 426nMAssay Description:Inhibition of human ErB2 phosphorylation in human SKBR3 cellsMore data for this Ligand-Target Pair