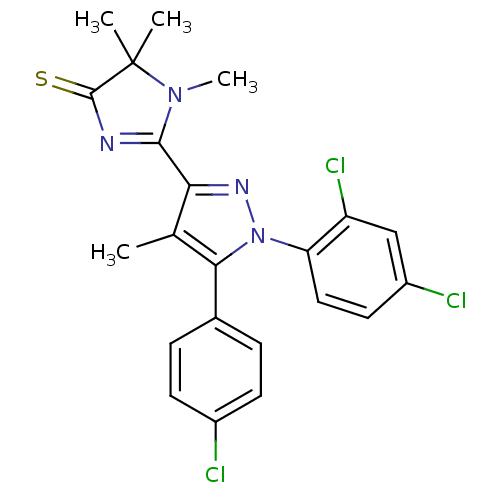
BDBM50279126 2-[5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-methyl-1H-pyrazol-3-yl]-1,5,5-trimethyl-1,5-dihydro-imidazole-4-thio::CHEMBL524634
SMILES CN1C(=NC(=S)C1(C)C)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl
InChI Key InChIKey=CYHSJMXZGJNACF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50279126
Found 3 hits for monomerid = 50279126
Affinity DataIC50: 6.30nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB1R expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 901nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2R expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 8.5nMAssay Description:Inverse agonist activity at human recombinant CB1R expressed in HEK293 cells assessed as inhibition of CP-55940-stimulated Eu-GTP bindingMore data for this Ligand-Target Pair