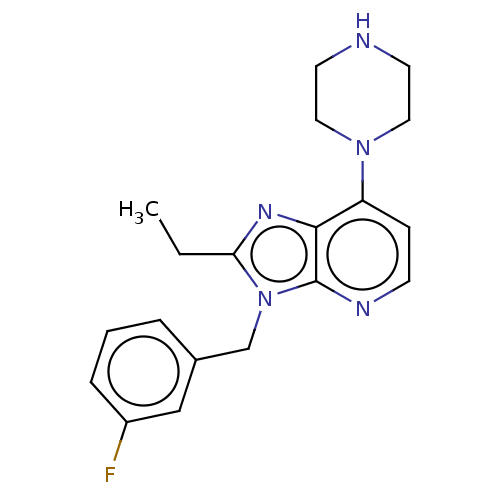
BDBM50291208 CHEMBL4175829
SMILES CCc1nc2c(ccnc2n1Cc1cccc(F)c1)N1CCNCC1
InChI Key InChIKey=VVFMFUGAHRAYJX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50291208
Found 8 hits for monomerid = 50291208
Affinity DataIC50: 18nMAssay Description:Antagonist activity at 5HT6R (unknown origin) transfected in NG108-15 cells co-transfected with CAMYEL assessed as reduction in cAMP levels after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Partial inverse agonist activity at 5HT6R (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]-LSD from human 5HT6R expressed in HEK293 cell membranes after 1 hr by microbeta counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to 5HT6R (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 441nMAssay Description:Displacement of [3H]-Ketanserin from human 5HT2AR expressed in CHO-K1 cell membranes after 1.5 hrs by microbeta counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.79E+3nMAssay Description:Displacement of [3H]-8-OH-DPAT from human 5HT1AR expressed in HEK293 cell membranes after 1 hr by microbeta counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.48E+3nMAssay Description:Displacement of [3H]-Raclopride from human dopamine D2L receptor expressed in HEK293 cell membranes after 1 hr by microbeta counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.23E+4nMAssay Description:Displacement of [3H]-5-CT from human 5HT7BR expressed in HEK293 cell membranes after 1 hr by microbeta counting methodMore data for this Ligand-Target Pair