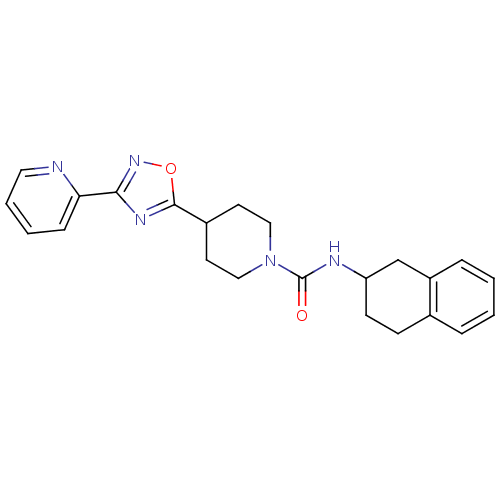
BDBM50295532 4-(3-(pyridin-2-yl)-1,2,4-oxadiazol-5-yl)-N-(1,2,3,4-tetrahydronaphthalen-2-yl)piperidine-1-carboxamide::CHEMBL550242
SMILES O=C(NC1CCc2ccccc2C1)N1CCC(CC1)c1nc(no1)-c1ccccn1
InChI Key InChIKey=BYFSZZCFOSXKJG-UHFFFAOYSA-N
Data 7 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50295532
Found 7 hits for monomerid = 50295532
Affinity DataIC50: 8nMAssay Description:Inhibition of human sEHMore data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acidMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of IKr channelMore data for this Ligand-Target Pair
TargetSodium channel protein type 5 subunit alpha(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Nav1.5 channelMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Cav 1.2 channelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human mEHMore data for this Ligand-Target Pair