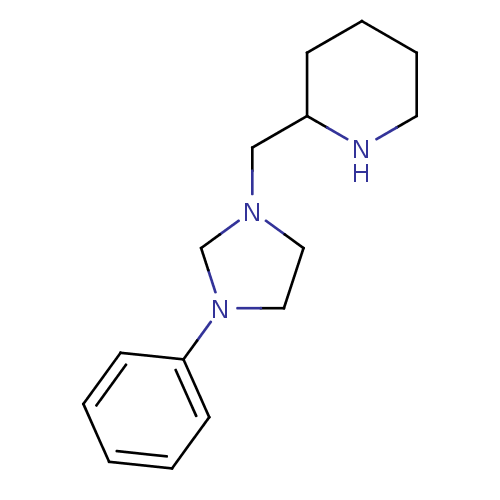
BDBM50313619 2-((3-phenylimidazolidin-1-yl)methyl)piperidine::CHEMBL1084132
SMILES C(C1CCCCN1)N1CCN(C1)c1ccccc1
InChI Key InChIKey=HVBAGGJDXDXSAY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50313619
Found 3 hits for monomerid = 50313619
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 by spectrofluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.58E+4nMAssay Description:Displacement of [3H]dofetilide from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.51E+3nMAssay Description:Activity at histamine H1 receptorMore data for this Ligand-Target Pair