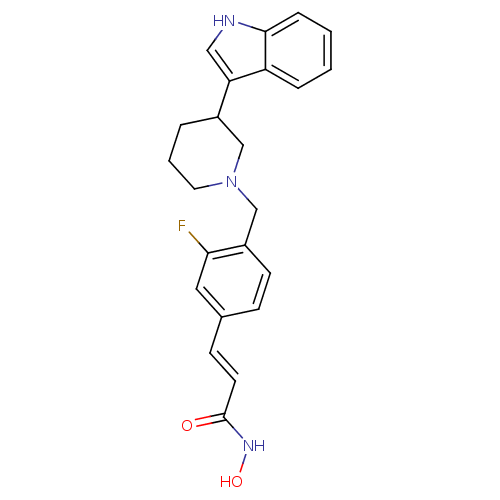
BDBM50314638 (E)-3-{3-Fluoro-4-[3-(1H-indol-3-yl)piperidin-1-ylmethyl]-phenyl}-N-hydroxyacrylamide::CHEMBL1093051
SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)c(F)c1
InChI Key InChIKey=CDODDYUNBWYGOC-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50314638
Found 2 hits for monomerid = 50314638
Affinity DataIC50: 136nMAssay Description:Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophysiology assayMore data for this Ligand-Target Pair