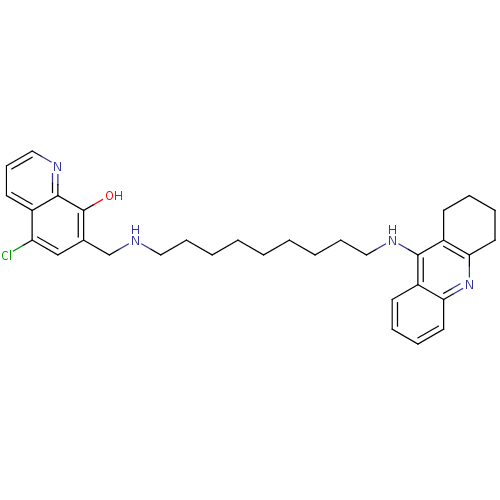
BDBM50322768 5-Chloro-7-{[9-(1,2,3,4-tetrahydroacridin-9-ylamino)nonylamino]methyl}quinolin-8-ol Dihydrochloride::CHEMBL1172762
SMILES Oc1c(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(Cl)c2cccnc12
InChI Key InChIKey=WBQRDKIQMPMEDD-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50322768
Found 4 hits for monomerid = 50322768
Affinity DataIC50: 1nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Inhibition of horse serum BChE by Ellman's reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibition of human serum BChE by Ellman's reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 85nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's reactionMore data for this Ligand-Target Pair