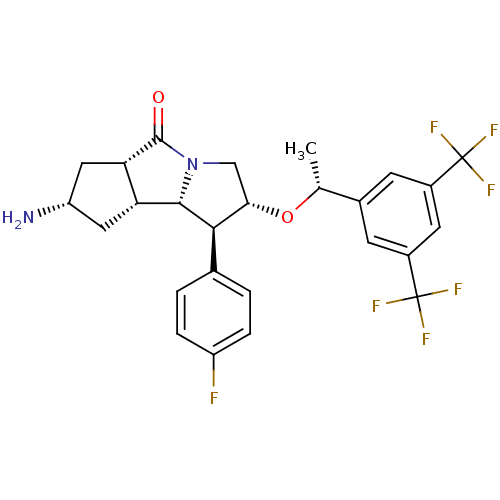
BDBM50327389 (1S,2R,5aS,7S,8aR,8bS)-7-amino-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-1-(4-fluorophenyl)octahydrocyclopenta[a]pyrrolizin-5(5aH)-one::CHEMBL1257235
SMILES C[C@@H](O[C@H]1CN2[C@@H]([C@@H]3C[C@H](N)C[C@@H]3C2=O)[C@@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F
InChI Key InChIKey=CMHQSZGZHCBTQY-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50327389
Found 3 hits for monomerid = 50327389
Affinity DataIC50: 0.0900nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human PXR inductionMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells in presence of 50% human serumMore data for this Ligand-Target Pair