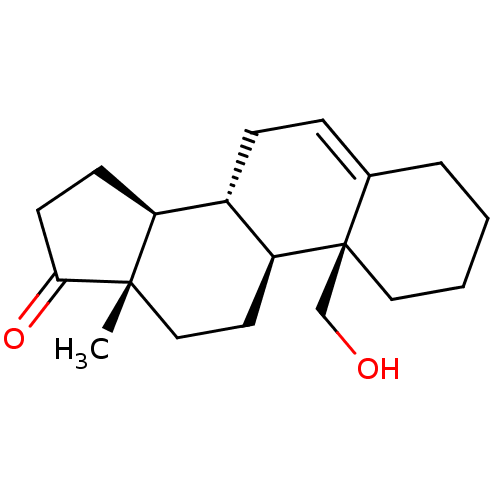
BDBM50332833 (8R,9S,10S,13S,14S)-10-(hydroxymethyl)-13-methyl-3,4,7,8,9,10,11,12,13,14,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one::CHEMBL1630911
SMILES C[C@]12CC[C@H]3[C@@H](CC=C4CCCC[C@]34CO)[C@@H]1CCC2=O
InChI Key InChIKey=VHEGBHRCHSDJHJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50332833
Found 3 hits for monomerid = 50332833
Affinity DataKi: 1.00E+3nMAssay Description:The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plotMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of human placental aromataseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:In vitro competitive inhibitory activity was measured on Cytochrome P450 19A1 of human placental microsomesMore data for this Ligand-Target Pair