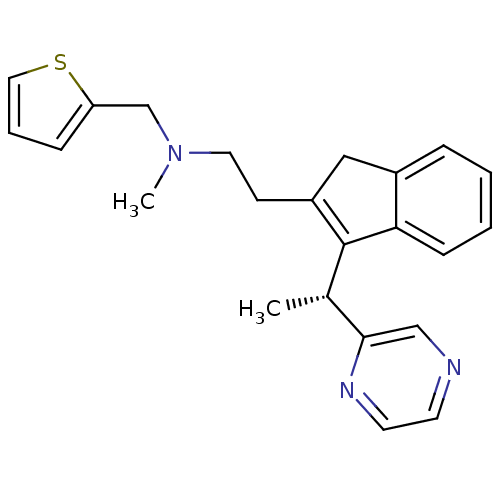
BDBM50336080 (R)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden-2-yl)-N-(thiophen-2-ylmethyl)ethanamine::CHEMBL1669419
SMILES C[C@H](C1=C(CCN(C)Cc2cccs2)Cc2ccccc12)c1cnccn1
InChI Key InChIKey=VVGNEGYIOFKVTA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50336080
Found 3 hits for monomerid = 50336080
Affinity DataKi: 6.70nMAssay Description:Binding affinity to histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.43E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair