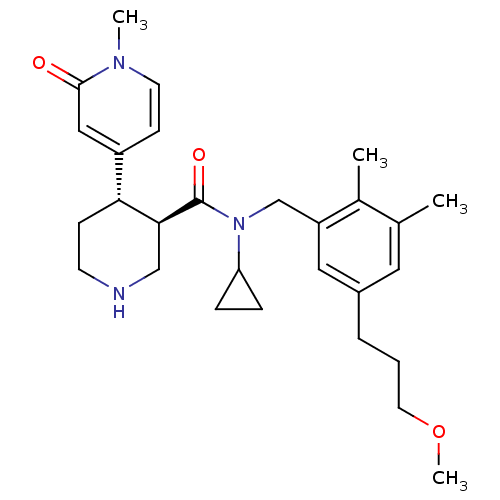
BDBM50347008 CHEMBL1796061
SMILES COCCCc1cc(C)c(C)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccn(C)c(=O)c2)c1
InChI Key InChIKey=LPGZXVGWFKRUJB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50347008
Found 3 hits for monomerid = 50347008
Affinity DataIC50: 0.600nMAssay Description:Inhibition of renin in human plasma using Q-FRET substrate pretreated for 10 mins before substrate addition measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of renin in human plasma using Q-FRET substrate pretreated for 10 mins before substrate addition measured after 1 hrMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: >2.90E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair