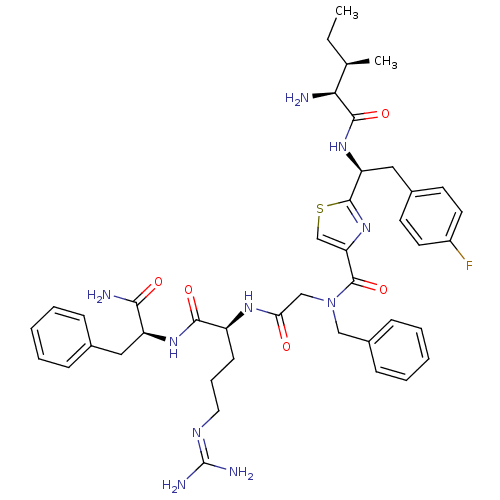
BDBM50366501 CHEMBL1790239
SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(F)cc1)-c1nc(cs1)-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#7])=O)-[#6]-c1ccccc1
InChI Key InChIKey=OCWQKEYRGVURQV-UHFFFAOYSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50366501
Found 1 hit for monomerid = 50366501
TargetProteinase-activated receptor 1(Human)
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of [3H]S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to a thrombin receptor (PAR-1) membrane preparation.More data for this Ligand-Target Pair