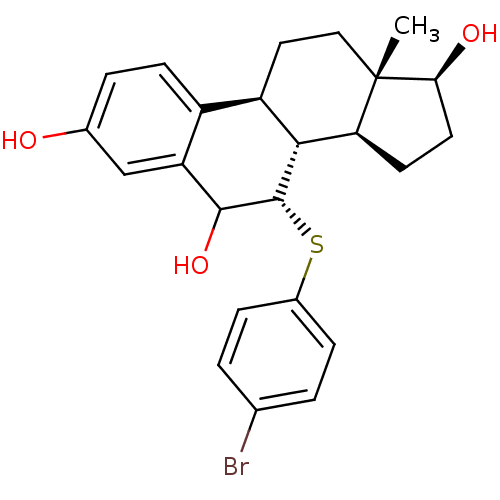
BDBM50366664 CHEMBL1627353
SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](Sc1ccc(Br)cc1)C(O)c1cc(O)ccc31
InChI Key InChIKey=XEKKTAYAEIHJEA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50366664
Found 3 hits for monomerid = 50366664
Affinity DataEC50: 7nMAssay Description:Agonist effect on transcriptional activation in MCF-7 cells expressing estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Displacement of [3H]17-beta-estradiol from human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 397nMAssay Description:Agonist effect on transcriptional activation in MCF-7 cells expressing estrogen receptor alphaMore data for this Ligand-Target Pair