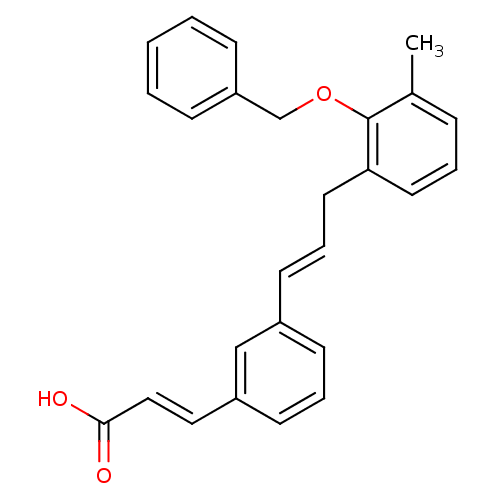
BDBM50370461 CHEMBL1237299
SMILES Cc1cccc(C\C=C\c2cccc(\C=C\C(O)=O)c2)c1OCc1ccccc1
InChI Key InChIKey=LQWBSXCGVBNXTL-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50370461
Found 4 hits for monomerid = 50370461
TargetProstaglandin E2 receptor EP2 subtype(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 630nMAssay Description:Binding affinity for human prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Binding affinity for human prostanoid EP3 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 3.10E+3nMAssay Description:Binding affinity for human prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.10E+4nMAssay Description:Binding affinity for human prostanoid EP1 receptorMore data for this Ligand-Target Pair