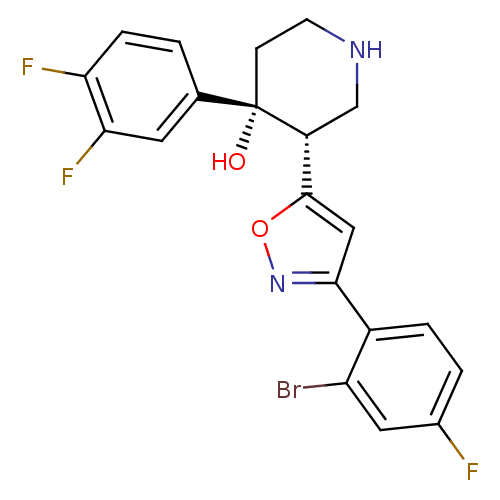
BDBM50380983 CHEMBL2016935
SMILES O[C@@]1(CCNC[C@@H]1c1cc(no1)-c1ccc(F)cc1Br)c1ccc(F)c(F)c1
InChI Key InChIKey=CXHZPUIYOWOMEL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50380983
Found 4 hits for monomerid = 50380983
Affinity DataIC50: 402nMAssay Description:Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate after 3 hrs by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.65E+3nMAssay Description:Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate pretreated for 10 mins measured after 3 hrs ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Reversible inhibition of CYP3A4-mediated conversion of testosterone to 6-beta-hydroxy-testosterone after 30 minsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Binding affinity to human ErgMore data for this Ligand-Target Pair