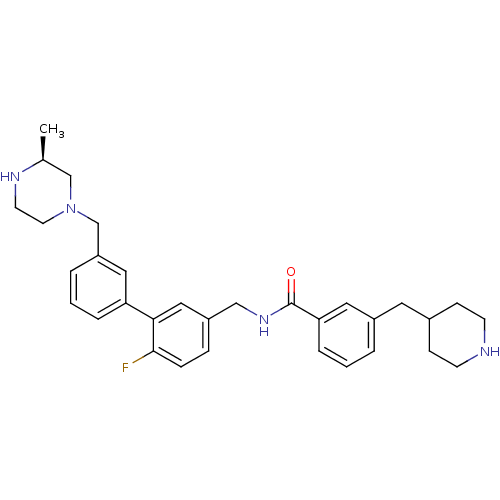
BDBM50412728 CHEMBL521523
SMILES C[C@H]1CN(Cc2cccc(c2)-c2cc(CNC(=O)c3cccc(CC4CCNCC4)c3)ccc2F)CCN1
InChI Key InChIKey=ZOZCBLGQKDOWFZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50412728
Found 10 hits for monomerid = 50412728
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataKd: 0.0100nMAssay Description:Antagonist activity at human recombinant muscarinic M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobili...More data for this Ligand-Target Pair
Affinity DataKd: 5.01nMAssay Description:Antagonist activity at human recombinant muscarinic M2 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobili...More data for this Ligand-Target Pair
Affinity DataKd: 0.200nMAssay Description:Antagonist activity at human recombinant muscarinic M1 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobili...More data for this Ligand-Target Pair
Affinity DataKi: 0.0316nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair