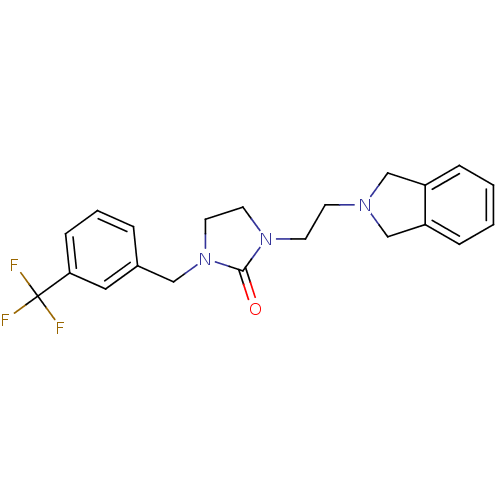
BDBM50414426 CHEMBL552366
SMILES FC(F)(F)c1cccc(CN2CCN(CCN3Cc4ccccc4C3)C2=O)c1
InChI Key InChIKey=PHZPOMMTJKRHHB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50414426
Found 3 hits for monomerid = 50414426
Affinity DataIC50: 6.31E+3nMAssay Description:Displacement of [3H]dofetilide from human ERG channel by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 12.6nMAssay Description:Antagonist activity at human dopamine D3 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 251nMAssay Description:Antagonist activity at human dopamine D2 receptor expressed in CHO cells by [35S]GTP-gamma-S-based scintillation spectrometryMore data for this Ligand-Target Pair