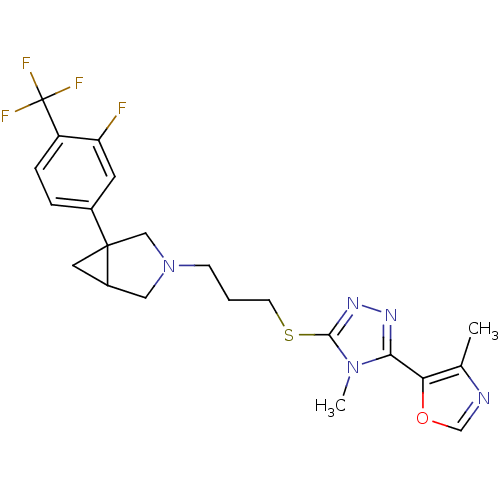
BDBM50415723 CHEMBL1080126
SMILES Cc1ncoc1-c1nnc(SCCCN2CC3CC3(C2)c2ccc(c(F)c2)C(F)(F)F)n1C
InChI Key InChIKey=MDDQIOXYIZYHFT-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50415723
Found 3 hits for monomerid = 50415723
Affinity DataIC50: 631nMAssay Description:Displacement of [3H]dofetidile from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 631nMAssay Description:Displacement of [3H]dofetidile from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 630nMAssay Description:Displacement of [3H]dofetidile from human ERG by scintillation proximity assayMore data for this Ligand-Target Pair