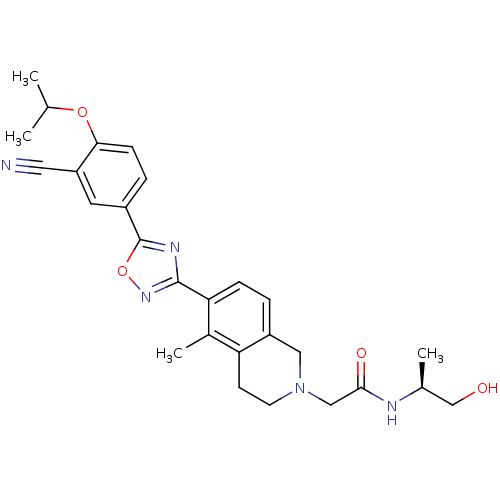
BDBM50419201 CHEMBL1836171
SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CC(=O)N[C@@H](C)CO)CCc2c1C
InChI Key InChIKey=VTHJWDKDWWJBFE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50419201
Found 3 hits for monomerid = 50419201
Affinity DataEC50: 1.26nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataEC50: 2.51E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair