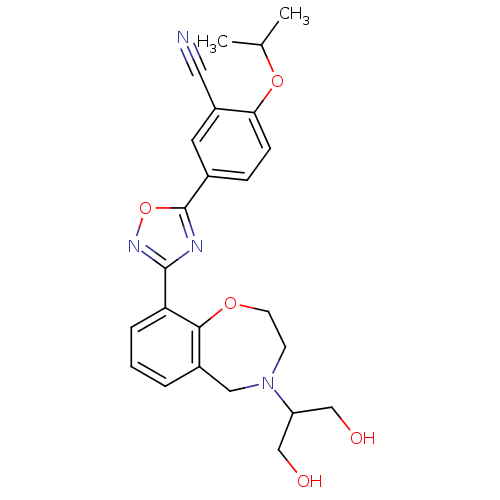
BDBM50419207 CHEMBL1836212
SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1cccc2CN(CCOc12)C(CO)CO
InChI Key InChIKey=KNSNIMFBLSWAKH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50419207
Found 3 hits for monomerid = 50419207
Affinity DataEC50: 19.9nMAssay Description:Agonist activity at human S1P1 receptor expressed in CHO-K1 EDG1 cells expressing beta-arrestin 2 after 105 mins by chemi-luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.76E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataEC50: >3.16E+4nMAssay Description:Agonist activity at human S1P3 receptor expressed in RBL cells assessed as [35S]GTPgammaS binding after 3 hrs by radiometric assayMore data for this Ligand-Target Pair