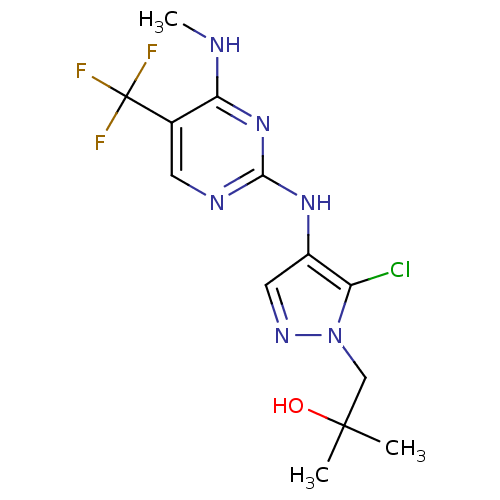
BDBM50426403 CHEMBL2326726
SMILES CNc1nc(Nc2cnn(CC(C)(C)O)c2Cl)ncc1C(F)(F)F
InChI Key InChIKey=IKBCWJODRQLGHR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50426403
Found 3 hits for monomerid = 50426403
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Charles River Discovery Research Services
Curated by ChEMBL
Charles River Discovery Research Services
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of full length wild-type LRRK2 (unknown origin) using biotinylated ezrin/radaxin/meosin peptide as substrate measured after 1 hrMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Charles River Discovery Research Services
Curated by ChEMBL
Charles River Discovery Research Services
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of LRRK2 (unknown origin) autophosphorylation by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Reversible inhibition of CYP1A2 (unknown origin) using phenacetin as substrate by LC-MS/MS analysisMore data for this Ligand-Target Pair