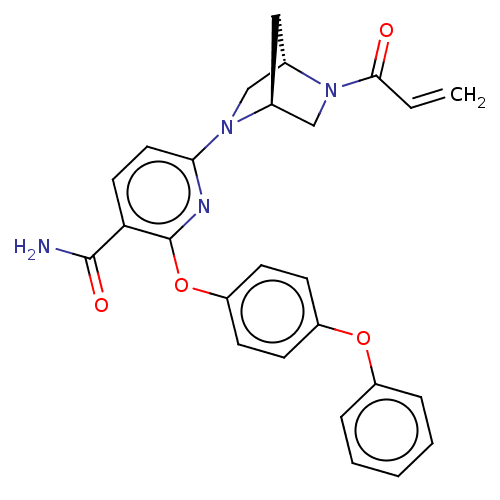
BDBM50463725 CHEMBL4242598
SMILES [H][C@]12CN(c3ccc(C(N)=O)c(Oc4ccc(Oc5ccccc5)cc4)n3)[C@]([H])(CN1C(=O)C=C)C2
InChI Key InChIKey=FVEYIFISRORTDD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50463725
Found 5 hits for monomerid = 50463725
TargetTyrosine-protein kinase BTK(Human)
Emd Serono Research & Development Institute
Curated by ChEMBL
Emd Serono Research & Development Institute
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Emd Serono Research & Development Institute
Curated by ChEMBL
Emd Serono Research & Development Institute
Curated by ChEMBL
Affinity DataIC50: 9.70nMAssay Description:Inhibition of BTK in human PBMC assessed as reduction in anti-IgM-induced CD69 expression incubated for 1 hr by flow cytometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Human)
Emd Serono Research & Development Institute
Curated by ChEMBL
Emd Serono Research & Development Institute
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of BTK in whole blood (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Emd Serono Research & Development Institute
Curated by ChEMBL
Emd Serono Research & Development Institute
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Binding affinity to human ERGMore data for this Ligand-Target Pair